The proportion of deuterium ("heavy hydrogen") in water trapped inside lunar rocks suggests it came from the same primitve-asteroid feedstock that supplied most of Earth's water.
The title of this post sounds like the beginning of a joke you might hear on The Big Bang Theory, but instead it's a tipoff that the geochemical evidence linking Earth and Moon is more robust than ever.
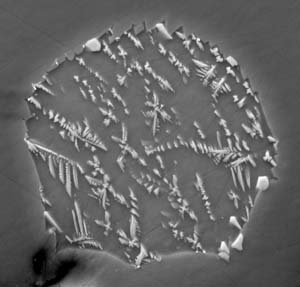
Backscatter electron image of a lunar melt inclusion (skeletal crystals) in an olivine crystal collected during Apollo 17. The inclusion is 30 microns across. Dark area at lower left is an experimental artifact.
J. Armstrong / Carnegie Inst. of Washington
The new insight comes from studies of primitive volcanic rocks returned from the Moon by the crews of Apollos 15 and 17. These contain tiny bits of glass trapped within olivine crystals, and those glassy bits (called melt inclusions) have a higher concentration of water — up to 1,200 parts per million (0.12%) — than any known lunar sample.
Alberto Saal (Brown University) and colleagues first found water in lunar material several years ago, and Saal again led the team whose analyses and conclusions appear in this week's Science Express.
They found the proportion of deuterium ("heavy" hydrogen that includes a neutron) in water trapped inside lunar rocks is close match to the deuterium-to-hydrogen (D:H) ratio in primitive meteorites called carbonaceous chondrites. Almost all of Earth's water must likewise have come from carbonaceous chondrites and other water-rich asteroids.
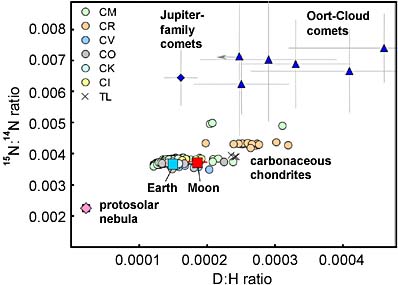
The ratio of deuterium (D) to hydrogen (H) is nearly identical in water from the Moon, Earth, and carbonaceous chondrites. Although at least one Jupiter-family comet has a comparable D:H ratio, the ratio of nitrogen isotopes in comets set them apart from those in inner-solar-system bodies.
A. Saal & others / Science Express
Decades ago, astronomers thought comets were the source of Earth's water, but in most cases their D:H ratio is too high, so comets contributed at most just a small fraction of what fills our oceans. While certain "Jupiter-family" (short-period) comets have Earth-like (and, now, Moon-like) D:H ratios, they can't have been major players because their nitrogen-isotope ratios are quite different.
The new finding means that lunar water likewise came from carbonaceous chondrites rather than comets. And since the Moon apparently formed from superheated matter splashed out during an enormous impact on Earth, the thinking now is that whatever water ended up inside the Moon must have come from Earth. In other words, our young planet was already wet (or at least very damp) when it got clobbered.
"The simplest explanation for what we found is that there was water on the proto-Earth at the time of the giant impact," Saal explains in a Brown press release. "Some of that water survived the impact, and that’s what we see in the Moon."
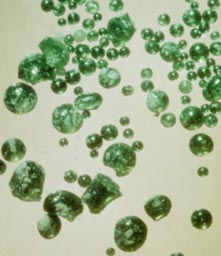
These small beads of green volcanic glass, collected by the crew of Apollo 15, contain a tiny trace of water.
NASA
These weren't easy measurements to make, notes co-author Erik Hauri (Carnegie Institution of Washington). That's because, once the lava flows reached the lunar surface, it started losing water molecules due to slow leaks from the rock itself and from molecular-scale damage caused by cosmic rays. These effects depleted hydrogen more readily than deuterium, artificially driving up the D:H ratio. So the researchers concentrated on water trapped in the melt inclusions, because it was protected from loss.
In candid moments, planet modelers will tell you that they need to figure out how the Moon managed to hang onto any water at all. But, that said, the realization that lunar water once resided on Earth strengthens the genetic link between the two worlds. In fact, evidence like this is forcing wholly new thinking about the type, mass, and velocity of the object that hit the young Earth. Specifically, it's becoming ever clearer that that the Moon assembled itself mostly at Earth's expense, not the impactor's.
 6
6
Comments
Bruce Mayfield
May 14, 2013 at 9:57 am
Usually these Earth – Moon formation news stories generate many comments. I guess people are in the process of adapting to the evidence that the Earth’s water supply is asteroidean and not cometary in nature, and perhaps the great collision formation of the Moon theory has reached a point where few want to question it. As to water from asteroids, researching “carbonaceous chondrites” lead me to discover that CM and CI chondrites contain from 3 up to a whopping 22 % water, so there could have been plenty of H2O from these and other space rocks to have supplied Earth’s hydrosphere, since water amounts to only about 0.023% of the Earth's total mass! The chart provided in Kelly Beatty’s article shows that the wetest of these metorites, the CI carbonaceous chondrites (color coded yellow) have isopic signatures that are quite Earth/Moon-like. The isotopic signatures do provide rather convincing evidence, but …
You must be logged in to post a comment.
Bruce
May 14, 2013 at 10:01 am
… what accounts for these isotopic differences? The chart plots the isotopic ratios of hydrogen against that of nitrogen, and it reveals an interesting trend in our solar system. In general you can see that as one moves further from the sun the abundances of the heavier isotopes for both H and N increase. Why would this be the case? Is this trend observed for other elements as well? This is not a leading question, I simply am puzzled by what seems to be counter-intuitive, since I would think that heavier atoms in our proto stellar cloud would have been sorted sunward, but the oposite evidently has happened. Why? If there is a good answer for this then the whole formation of the solar system question would be on a firmer foundation, but if this isotopic abundance question is still unexplained then any conclusions that could be drawn therefrom would be questionable too.
You must be logged in to post a comment.
Peter
May 15, 2013 at 1:10 pm
Good question. The same physical process will often go one way in one situation, and the other way in another. For example, “tidal interaction” causes Mars’ Phobos to spiral inward and Deimos to spiral outward. Evaporation will favor the lighter isotopes; condensation the heavier. Which process prevailed where and when? Also, molecular isotopes have a higher EM cross-section than the lighter ones. E.g. HD’s rotational and vibrational modes are closer together than H2’s, and there are more of them, so HD will absorb more infrared than H2. This effect may contribute. There may be no simple answer.
You must be logged in to post a comment.
Bruce
May 16, 2013 at 4:33 pm
Interesting answer Peter. Chemically isotopes of the same element usually behave the same, but not always. I’ve been attempting to learn about how isotopic mass fractionation can or has occurred in our solar system. Using the search feature on this site led me to find another fine K. Beatty article from 5 years ago; http://skyandtelescope.org/community/skyblog/newsblog/16593651.html
This article discussed oxygen isotope ratios of the solar wind that were sampled by the Genesis mission. The finding was that the solar wind contains ‘far more O16, relative to O17 and O18, than is present in terrestrial seawater.’ Comments about this by Grant Martin and confirmed by Oliver K. Manuel showed that, “Mass fractionation in the Sun selectively moves lightweight elements (H, He, C, etc) and lightweight isotopes of each element (O16, Ne20, Mg26, … Xe124, etc.) into the photosphere and then into the solar wind.” Since the solar wind diminishes with distance from the Sun and since the solar wind is preferentially loaded with lighter isotopes, I now believe that the sun and the solar wind are the main reasons for the isotopic differences observed across the solar system. Sometimes yesterday’s deep mystery is today’s wow, I should have thought of that!
You must be logged in to post a comment.
Peter
May 18, 2013 at 9:00 am
Well, that's one of us that understands it!
You must be logged in to post a comment.
Bruce
May 18, 2013 at 9:37 pm
Of course, it could be that I just think that I’ve got a handle on it Peter, and I really don’t understand it at all. It just seems logical that sorting of light elements and isotopes could have occurred in the very young Sun, and when it goes through the intense solar wind T-Tauri phase the light isotope laden wind loads objects close to the sun with light isotopes. The further an object is from the Sun the less light isotopes are received, accounting for the observed trend.
You must be logged in to post a comment.
You must be logged in to post a comment.