Once Phoenix gets down and dirty with its surroundings, one of its first scientific tasks will be to determine the salt content of samples of Martian soil gathered by its robotic arm.

On May 27, 2008, one "sol" (Martian day) after landing, NASA's Phoenix spacecraft recorded this view of the landscape looking northwest. The polygon-textured terrain, also visible from orbit, is typical of polar environments on Earth. Click here to see a larger version and here to see it in full resolution.
NASA / JPL / Univ. of Arizona
The experiment that will do this, called the Microscopy, Electrochemistry, and Conductivity Analyzer (MECA) contains a miniaturized wet-chemistry laboratory with four teacup-size beakers.
We already know that salts exist on Mars. The rover Spirit found evidence for ancient hot springs, and its twin Opportunity identified salts in the rocks and dirt along its route. Meanwhile, spectrometers aboard the Mars Odyssey and Mars Express orbiters have found the spectral fingerprint of salts all over the Red Planet, and some Martian meteorites contain salts as well.
So if water once flowed on Mars, it was probably briny. That, in itself, wouldn't prohibit the existence of life. Biologists have a long list of environments on Earth that teem with halophilic ("salt-loving") microorganisms. The next time you visit Utah's Great Salt Lake or the Dead Sea in the Middle East, bring your microscope.
But there comes a point when even the hardiest halophiles give up, and a trio of scientists have determined that Mars might be too salty for life to have survived, let alone thrived. The new result, published in this week's Science, finds that ancient brines on Mars were typically 10 to 100 times more saline than terrestrial seawater. And that's not even figuring in the sulfates, which appear to be the dominant salt type on the Red Planet.
To assess the habitability of Martian soils, Harvard researcher Nicholas Tosca and his colleagues assigned a "water-activity" value that indicates how suitable a solution is for use by organisms. Pure H2O has a value of 1.0, seawater is 0.98. Only a few halophiles manage to hang on below 0.85.
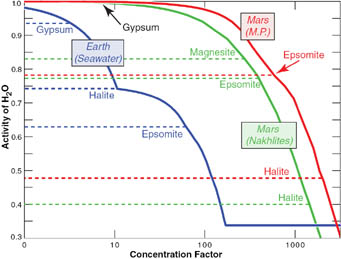
Based on chemical assays of Martian soils at Meridiani Planum made by the rover Opportunity (red curve) and of Martian meteorites (green curve), brines once present on the red Planet were likely far saltier than the seawater in our oceans (blue curve). This implies that the "activity" of Martian water — its availability for biological processes — was too low to sustain organisms. Click on the image for a larger version.
Science / Nicholas Tosca & others
By contrast, the team believes brines on the surface of ancient Mars had water activities that likely ranged from 0.78 to 0.86 — plunging to 0.5 or 0.6 as evaporation caused the brines to become more concentrated.
The abundant water ice thought to lie beneath Phoenix might be relatively pure, because it precipitated from the atmosphere as snow or frost. But abundant salts could still be present, layered within the ice as fine particles carried to the poles by the planet's ferocious dust storms. And water-activity values drop steadily at temperatures below freezing.
"We'll have to wait and see what Phoenix finds," Tosca cautions. "Our paper simply says that knowing something about the surface chemistry on Mars is important in judging its habitability."
You can't access the full Science article without a subscription, but this Harvard press release highlights the salient details.
 2
2
Comments
P. Livingstone Hanley
May 30, 2008 at 12:44 pm
I am aware that most metals, when exposed to salt, salt water or salty air corrode very rapidly. Have the Martian exploration vehocles shown any sign of srious corrosion from being exposed to salty martian soil? Perhaps if they do not show signs of corrosion could it be that it is because of the lack of moisture (water)? Would moisture (water) need to be present to "activate" the salt corrosion?
You must be logged in to post a comment.
Fred from Laurel, Md
June 8, 2008 at 7:34 pm
Correct me if I'm wrong, but doesn't water- or saltwater-induced corrosion require the presence of oxygen or some other oxidizer, so that the (salt)water is only a catalyst? That is, unless the metal is so reactive (Li, Na, Mg, K, Ca, etc.) that it reacts with the O in the water, which, of course, isn't the case for the metals in the lander.
You must be logged in to post a comment.
You must be logged in to post a comment.