When English poet Samuel Taylor Coleridge penned "Water, water everywhere, and not a drop to drink" more than 200 years ago, he could not possibly have foreseen how aptly this now-famous line might describe the polar plain where NASA's Phoenix spacecraft touched down last year.
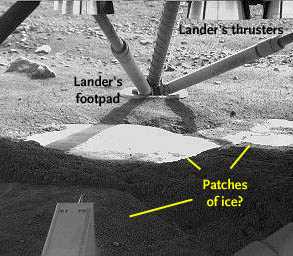
The Robotic Arm Camera on NASA's Phoenix Mars Lander captured this image underneath the lander, showing smooth surfaces cleared from overlying soil by the rocket exhaust during landing. The two level, overexposed surfaces in the center, plus the shadowed one at lower left, were scoured clean by rocket exhaust and are almost certainly exposures of water ice.
NASA / JPL / Univ. of Arizona / Max-Planck Inst.
The robotic prospector went looking for Martian ice and found it in abundance. A camera on the craft's robotic arm glimpsed slabs of ice directly beneath the main chassis. Apparently the lander's descent rocket had scoured away a few inches of loose grit to expose the icy bedrock. However, repeated attempts to deposit a sample of ice into any of several instrumental hoppers failed. The rock-hard slab proved difficult to scrape, and the clumpy soil above it clung tenaciously to the sampling scoop or clogged the instruments' inlets with unyielding clods.
Yet the Phoenix scientists caught an unexpected break. The superheated exhaust also splashed small bits of mud onto the craft's landing struts — little beads of material that grew, merged, and even dripped over the next few weeks. Those dynamic blobs must have been drops of concentrated saltwater, concludes a large scientific team led by Nilton Renno, a Phoenix investigator at the University of Michigan.
Renno presented his case today at a meeting of planetary scientists near Houston, Texas. Liquid water would hardly be expected at the north-polar landing site, where the temperature never climbed above -5°F (-20°C) during five months of operation. For example, images showed that whenever the lander's mechanical scoop unearthed fresh exposures of ice, it quickly sublimated (vaporized) directly into the atmosphere.

Droplets on a leg of the Phoenix lander enlarge and coalesce in this image sequence taken on sols (Martian days) 8, 31, and 44 following the craft's arrival in the north polar region of Mars last year.
NASA / JPL / Univ. of Michigan / Max Planck Inst.
The key to keeping water from freezing, Renno explained, was the discovery that the silty, clumpy soil in the landing zone contains abundant chlorine-rich salts called perchlorates. Through a process called deliquescence, the salty spatter on the landing strut absorbed enough water vapor directly from the thin Martian air to liquefy. These droplets of concentrated brine freeze at temperatures some 125°F (70°C) lower than that for water alone.
According to Renno, the droplets grew in size at just the right rate to match theoretical predictions. This growth diluted the salt content and made them more susceptible to nighttime freezing. After some six weeks, the beads' enlargement stopped, suggesting that they'd reached balance with the relative humidity of the surrounding air. Toward the mission's end, they shrank somewhat, a sign that the droplets were slowly desiccating.

Supersonic exhaust from the Phoenix lander's braking rockets had a temperature of more than 1,800°F (1,000°C).
NASA / JPL / Univ. of Arizona
Although scientists are now convinced that liquid water must have once existed on the Red Planet, it's a surprise to find it in one of the planet's coldest environments. Emboldened by this discovery, Renno now believes that supersalty brines might be common on Mars.
Does this mean present-day has suddenly blossomed into an abode for life? Not exactly, though in a follow-up presentation astrobiologist Carol Stoker (NASA-Ames Research Center) rated the Phoenix site as far more bio-friendly than any other Martian landing site to date.
Read more about the case for liquid Martian saltwater in this University of Michigan press release.
 1
1









Comments
Christopher Staffa
March 29, 2009 at 10:55 am
Water, water, every where,
Nor any drop to drink.
You must be logged in to post a comment.
You must be logged in to post a comment.